What We Do
Mechatronics Design
The core competence of Mediagnost is development of complex system solutions in the field of mechatronics from idea till serial production, through valuable concept designs and customer specifications. PTC Creo environment based on DfX methods and supported with FEM engineering calculations ensures cost effective detailed design.
Development risk mitigation is managed by V-shape design process in conformance with IEC60601 and FDA requirements. Mediagnost provides DMR, DHR maintenance and configuration control. Device History File can be maintained in PTC Windchill PLM System. We are capable to design motion controller PCBAs, serial production tooling and functional test equipment (HW+SW development).


Industrialization
We offer our customers turnkey ready project management: after the approved design-out we care about the entire industrialization. With a wide scope we continuously improve performance, productivity and quality of our products by identifying problems, bottlenecks, waste, and risks. We are ISO9001, ISO13485 and ISO14001 certified and know how to handle specific documentation demands in the medical sector. We support our customers with quality tools as APQP, SPC, Capability Analysis, MSA, Gauge G&R, etc. No matter if CoCs and CoAs are required we are glad to serve You with these documents through our integrated ERP System.









Manufacturing
- Wide range of manufacturing capabilities in-house
- Up to 5 Axis turning, milling
- Gear hobbing
- Wide range of sheet metal processing
- Diverse kinds of welding
- Lead processing
- Gluing
- Covers from plastics
- Paint shop
- Cable assembly and testing
- 3D Printing (FDM)

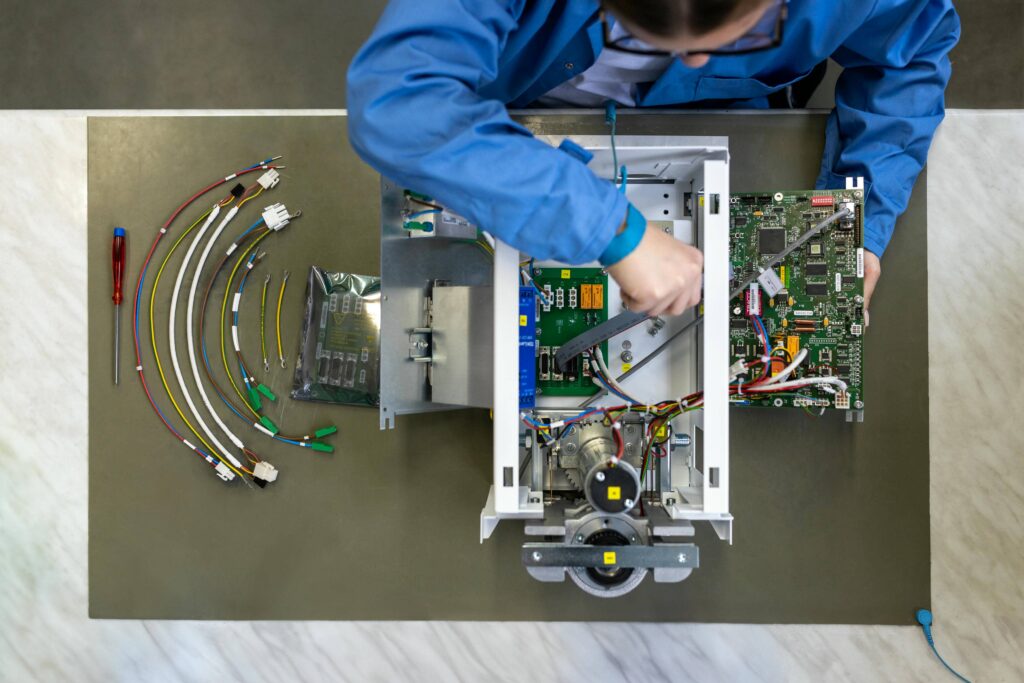
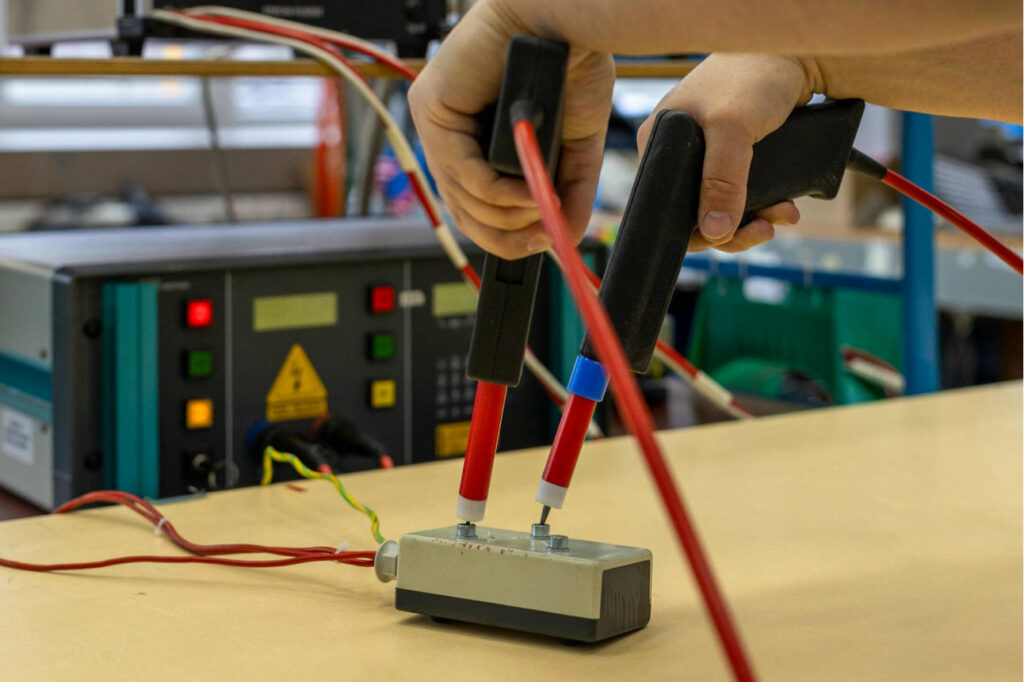


Integration and Testing
Complete modules from diverse mechanical and mechatronics components, cables, electronics are assembled in well-engineered and highly controlled processes. Assembly and integration are supported by owned design, optimized tools, jigs and functional testing equipment. Full traceability is part of the standard procedure. We have tracked data of all our high mix components from the first source any beyond, through manufacturing processes all the way to our finished product. This enables us a safe and responsible manufacturing process.

Supply Chain management
We organize, manage and optimize the complete value chain with worldwide supply base. We take care of support activities such as supplier selection and evaluation, purchasing, stock management, physical distribution of your product even up to service life spare part supply. Customer specific logistical solutions such as Vendor-Managed Inventory (VMI) are applicable if required
We manage more than 600 suppliers from all over the world. Our customers based on their own preferences can predefine suppliers. Our own supply base is selected based on required capabilities, quality, costs and trust. With our suppliers, we achieve a win-win situation. Close collaboration with our suppliers is a key factor of our success and part of our company philosophy.

Life Cycle management
Life cycle management starts at Mediagnost already with the FUMOs and finishes at the end of service lifetime of the product. To carry these activities we have solid processes and integrated system for a comprehensive change control. No matter if components or productions technologies may not available at a later date. We use optimal strategies for the benefit of our customers in order to keep products in the market for many years.
Our References
Our current customers are Europe’s biggest and the world most famous Medical Device Manufacturers.
For these clients we designed and manufacture complete X-ray mechatronics
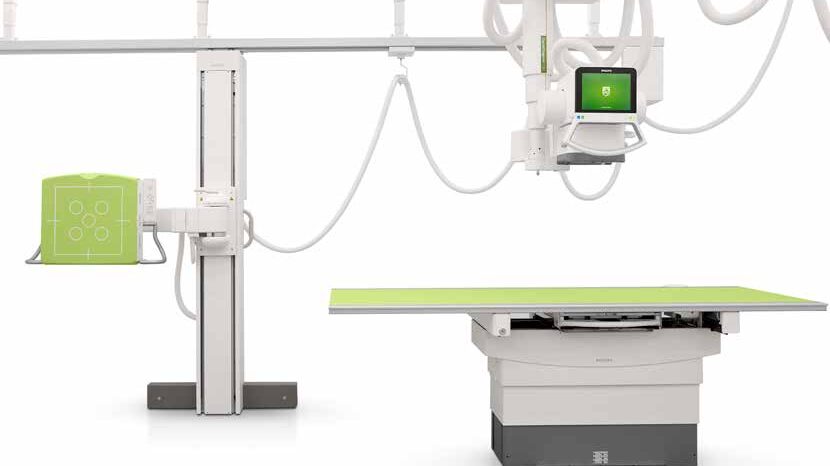
Philips Medical Systems

Philips DXR
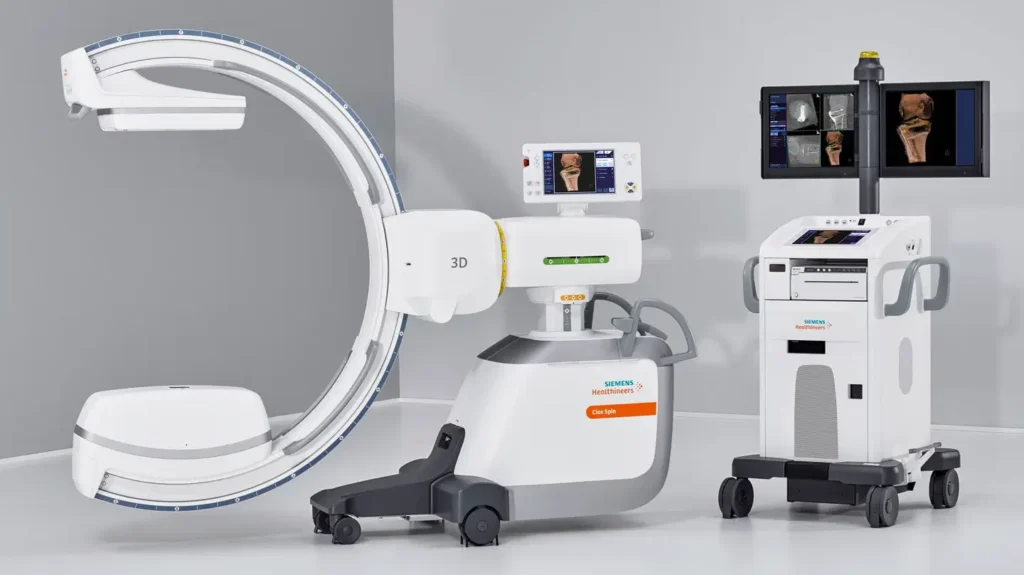
Siemens Healthineers
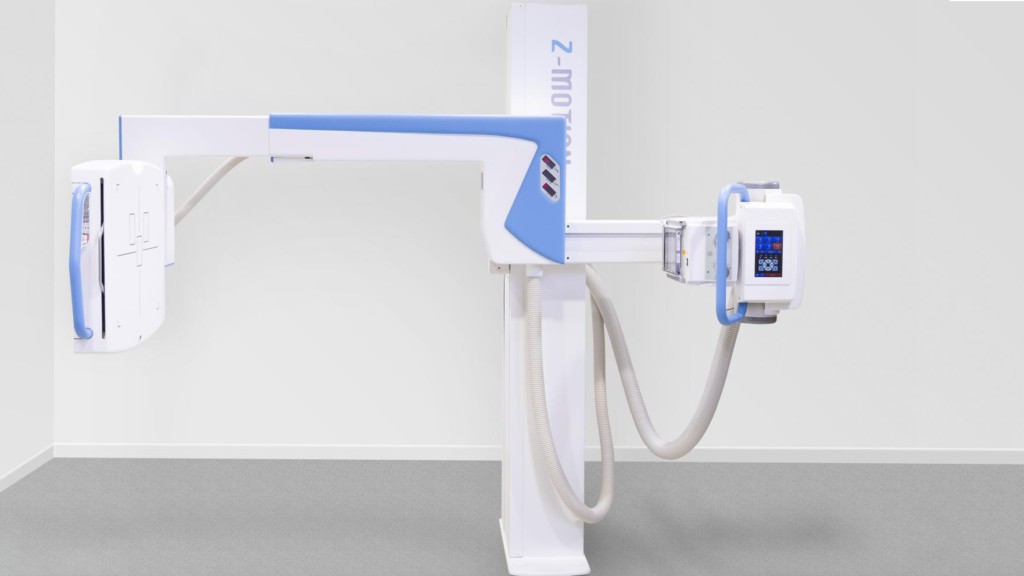
Innomed Medical

Control-X
Mediso